For a cosmetic product notification in Malaysia there must be a locally incorporated company ie License Holder or a Cosmetic Notification Holder CNH which can act as a single point of contact on behalf of the. This makes the cosmetic product registration Malaysia an extremely tedious process with possibilities to affect the ROI and the time taken for the procedures.

Registering A Cosmetic Product In Malaysia Azmi Associates
No person shall manufacture sell supply import possesses any cosmetics unless the.

. Cosmetic Product Registration in Malaysia. 19 May 2021. Under the Control of Drugs and Cosmetics Regulation 1984 Regulation 18A 1.
Cosmetic products in Malaysia are generally regulated under Sale of Drugs Act 1952 to be read together with Control of Drugs and Cosmetic Regulations 1984 CDCR 1 and Guidelines for Control of Cosmetic Products in Malaysia Guidelines. Lot 36 Jalan Universiti 46200 Petaling Jaya Selangor. The applicant for product registration for pharmaceutical products is known as the Product Registration Holder PRH.
Offering not only regulatory services with reasonable and competitive price also we are expertise in custom clearance trademark registration solving all your problems in one shot and hassle no more. Cosmetic products in Malaysia are generally regulated under Sale of Drugs Act 1952 to be read together with Control of Drugs and Cosmetic Regulations 1984 CDCR1 and Guidelines for Control of Cosmetic Products in Malaysia Guidelines. Data to be communicated.
This process can oftentimes be quite confusing especially if you are a foreign brand looking to expand your business in Malaysia. Be it a problem with importing your products to corresponding. Mark Your Products Safe.
Cosmetic products in Malaysia are generally regulated under Sale of Drugs Act 1952 to be read together with Control of Drugs and Cosmetic Regulations 1984 CDCR1 and Guidelines for Control of Cosmetic Products in Malaysia Guidelines. As such each company is required to notify NPRA before it can sell manufacture and distribute any cosmetic products. Product notification for cosmetic product is only limited to companies that are duly incorporated or registered with Syarikat Suruhanjaya Malaysia or Malaysian Registrar of Business.
Control of Drugs and Cosmetic Regulations CDCR 1984. Cosmetic Notification Holder CNH. NOT Token COA MicroB and simplify heavy metal testing are part of the registration needed.
Hence we have streamlined the process to deliver a seamless experience throughout your journey with us. Cosmetic Product Registration in Malaysia. For cosmetic product registration the online registration procedure for all cosmetic products has been substituted by the online notification procedure as of 1.
Location map Phone. Under CDCR a company which intends to manufacture sell supply import and possess any cosmetic product must. Drop us a Call Our team are glad to serve you daily.
The Product Registration Holder will then be responsible for all matters relating to product quality safety and efficacy. Under CDCR a company which intends to manufacture sell supply import and possess any cosmetic product must. Who can register.
A PRH must be a locally incorporated company corporate or legal entity with permanent address and registered with Companies Commission of Malaysia with the scope of business related to the healthpharmaceutical product. TECOLABs well-experienced product registration team is readily available to assist you in your cosmetic product registration in Malaysia. MM cosmetic provides NPRA product registration for our client to register their OEM ODM or OBM products with NPRA product registration with the authority.
We register products such as creams emulsions lotions gel oil for skin face masks make-up powders after bath powders hygienic powders toilet soaps deodorant soaps perfumes bath or shower preparations deodorants anti-perspirants hair care products shaving. The CNH may or may not be the proprietor of the product. Furthermore products for making-up removing make-up from the face the eyes.
Malaysias Drug Control Authority DCA which was established under the Control of Drugs and Cosmetics Regulations 1984 is a government authority that ensures the safety effectiveness and quality of cosmetic products marketed in Malaysia. The DCA is responsible for the registration and licensing of all. Since only Malaysian companies may apply for product registration a foreign company will have to appoint a Market Authorisation Holder MAH as their local representative to obtain product registration.
Cosmetic registration is a process that brands wishing to sell their products in Malaysia are required to go through in order to ensure that their products are marked safe to use. In agreement with the harmonisation of cosmetic through the ASEAN Cosmetic Directive ACD cosmetic products in Malaysia are controlled through notification procedure starting from. For Use In Malaysia.
Under CDCR a company which intends to manufacture sell supply import and possess any cosmetic. If a foreign company intends to bring in cosmetic products into Malaysia they will first be required to appoint a local agent a company registered in Malaysia to be the holder. To become the owner of the registration certificate a foreign corporation that wants to introduce pharmaceutical product registration into Malaysia first has to find a local agency a company registered in Malaysia.
Although the process of registration is detailed in the official guidelines rapid changes and communication barriers are often challenges faced by local and foreign companies. Cosmetic products in Malaysia are regulated under the Control of Drugs and Cosmetic Regulations CDCR 1984 which were promulgated under the Sale of Drugs Act 1952. Cosmetics-related locally incorporated company or legal entity with a permanent address and registration with the Malaysian Companies Commission.
According to the National Pharmaceutical Regulatory Agency NPRA cosmetic products are substances that are intended to be placed in contact with various external areas of the human. We offer advisory and consultancy services for obtaining. Commonly known as Cosmetics Product Registration Cosmetics products are regulated under the Control of Drugs and Cosmetics Regulation 1984 which were promulgated under the Sale of Drugs Act 1952.
Since 1st January 2008 cosmetic products in Malaysia are controlled through notification procedure. The requirements and procedure to register the cosmetic products shall be as follows. There are few certificates or registration needed for a new product to be able to sell in Malaysia market.

Pharmaboardroom Regulatory Pricing And Reimbursement Malaysia

Chemlinked Cosmetic Regulatory Market Intelligence Expertise In Ap Especially China

Cosmetic Product Notification Who Collaborating Centre For Regulatory
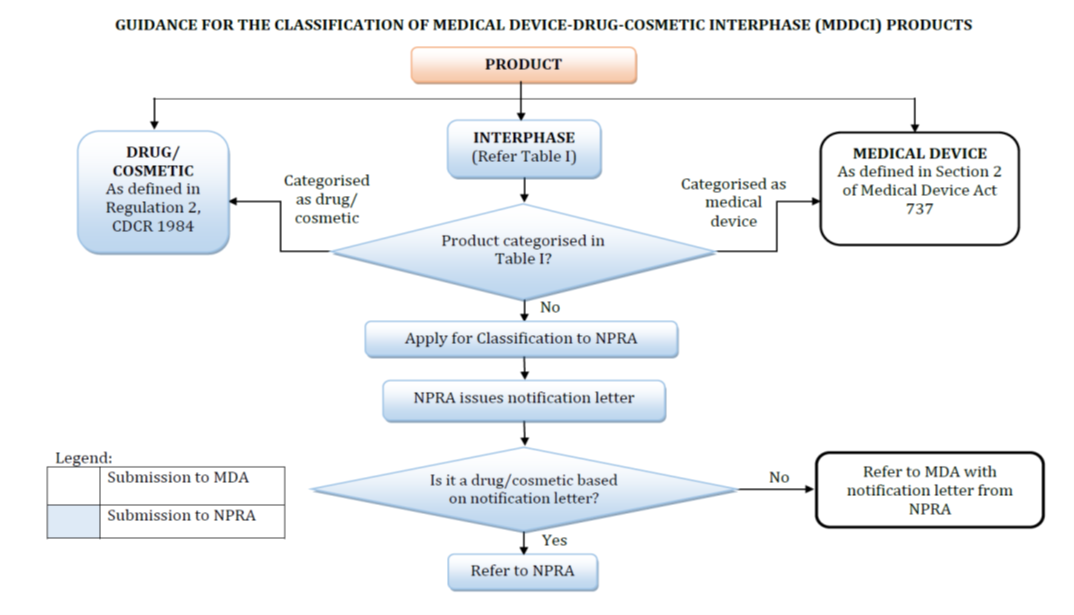
Product Classification Guideline Medical Device Drug Cosmetic Interphase Products

How To Achieve Malaysia Halal Cosmetics Compliance In Cosmetics Connect

Pdf Regulation Of Cosmetics What Has Malaysia Learnt From The European System

Cosmetics Manufacturer Malaysia What You Need To Know

Malaysia Cosmetic Regulation Guide Cosmetics Bridge

Registering A Cosmetic Product In Malaysia Azmi Associates
Registering A Cosmetic Product In Malaysia Legal Developments

Pdf Legal Control For The Safety Of Cosmetic Products Application Use In Malaysia

Registering A Cosmetic Product In Malaysia Azmi Associates

Cosmetic Manufacturer And Supplier Company In Selangor Malaysia

Malaysia Cosmetic Regulation Chemlinked

Malaysia Cosmetic Regulation Guide Cosmetics Bridge

Cosmetic Product Notification Who Collaborating Centre For Regulatory

Malaysia Cosmetic Regulation Chemlinked
Pdf Review On Aesthetic Cosmetic Industries In Malaysia A Way Forward

Npra Get Your Pharmaceutical Cosmetic Other Products Registered In Malaysia National Pharmaceutical Regulatory Agency
